
Working group
Biobanks
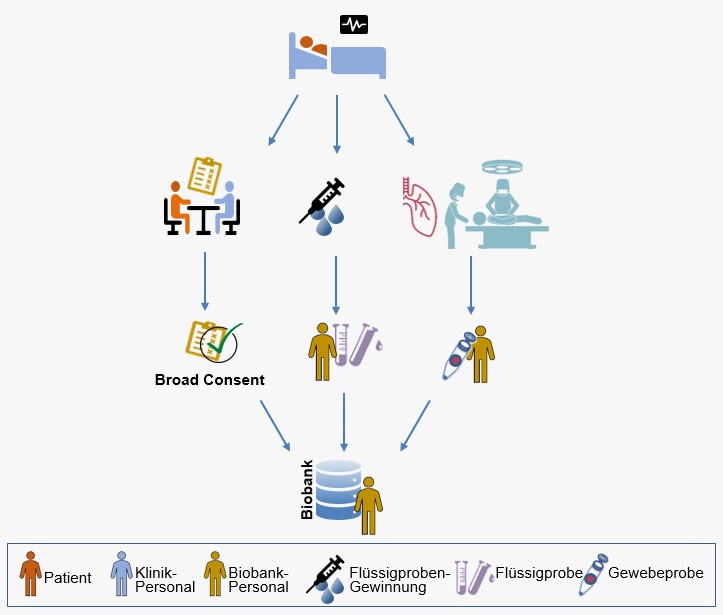
The availability of biomaterials is an indispensable condition for translational, application-oriented cancer research. The systematic collection and preservation of these materials in biobanks is, therefore, a key concern of the Bavarian Cancer Research Center (BZKF).
Activities & Achievements
The availability of biomaterial and associated data is an indispensable condition for translational research, not only in hematology, but for a multitude of scientific questions in cancer research. The members of the working group Biobanks try to establish a set of harmonized standards in order to enable cross-site cooperation in the BZKF network. Studies have shown that one third of non-reproducible experimental analyses are due to poor sample quality. Because of this, a maximum quality standard realized uniformly across all centers is one of the leading goals that are being pursued. Other points pursued in the working group are the definition of equipment standards and a common solution regarding data protection, informed consent and data exchange.
Other tasks/basic requirements include
- Facilitate access and sharing of materials/data between sites through close networking.
- Development and harmonization of an extended oncology data set to enable cross-site use of the data for research groups.
- Establishment of a common data center for the six participating biobanks.
- Close networking with other working groups and lighthouses of the BZKF conceptual development and transfer to clinical application.
- Conceptual development and alignment of corresponding structures within the BZKF biobanks (e.g. premises, personnel resources, medical-technical equipment, etc.)
Long-term goals at all BZKF sites
- Harmonization and close networking between the six biobanks
- Alignment of the basic equipment of the biobanks
- Definition and harmonization of Standard Operating Procedures for biobanks
- Standardized and harmonized data processing
- Implementation of a uniform and comprehensive patient consent / “Broad consent”
- Implementation of self-initiated studies
WORKING Group leader:
Prof. Dr. Martin Trepel University Hospital Augsburg
Prof. Dr. Bruno Märkl University Hospital Augsburg
