
The ECTU Tumor Board - better access to early clinical trials
The number of clinical trials in Germany is continuously decreasing, particularly in the early phases of drug development. Strict regulatory requirements, high data protection requirements, staff shortages and an increasing documentation burden make it difficult to conduct such studies. This poses an enormous challenge for doctors: the search for suitable studies for their patients is time-consuming and often lacks transparency.
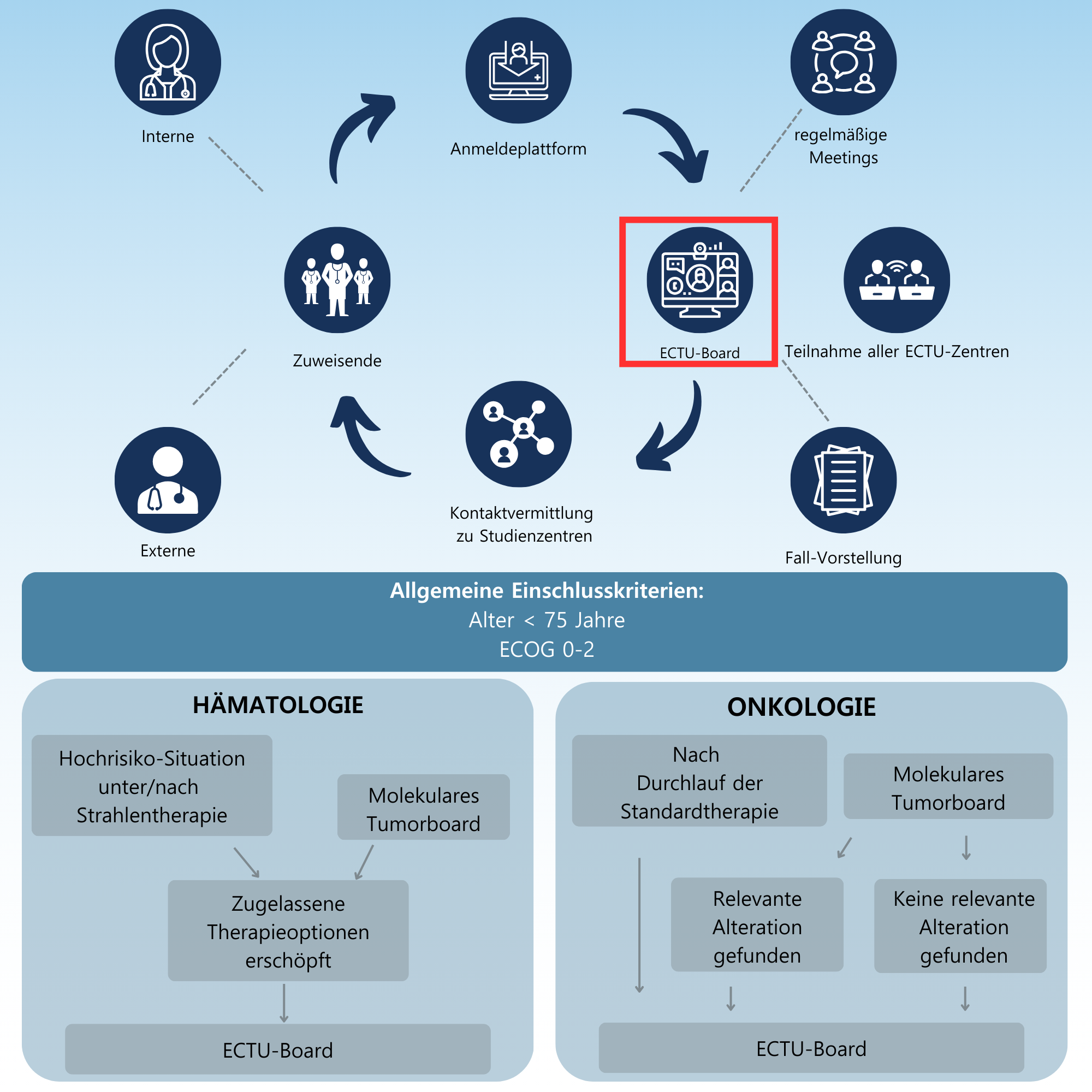
The ECTU Tumor Board was created to close this gap. It offers a cross-location network that makes it easier for patients to access innovative therapeutic approaches. Treating oncologists can present patients here for whom there is no suitable study at their own location. Experts from various national Early Clinical Trial Units (ECTUs) exchange current information about ongoing and planned trials in this virtual forum. This enables patients to be included in suitable trials more quickly and in a more targeted manner.
The Tumor Board was initiated in Munich in 2021 and is now supported by the BZKF and German Cancer Aid. Since March 2024, there has also been a separate tumor board for pediatric patients. The continuous expansion of the network, also beyond Bavaria, enables better care across the board.
Interdisciplinary and virtual
Tumor conferences usually take place on an interdisciplinary basis within a hospital. New data protection concepts have to be developed for a tumor board between several locations. A platform was developed specifically for the ECTU tumor board to ensure reliable communication of patient information. This is a certified and secure website that the representatives of the ECTUs can access. At LMU Klinikum München, a concept was developed together with the responsible data protection officer in order to adequately protect the data of the registered patients and still enable an exchange between the various locations. The project was rolled out together with the representatives of the Bavarian university hospitals in Würzburg, Erlangen, Regensburg, Augsburg and Munich (Technical University of Munich). Patient cases are discussed virtually once a month. Since the integration of the Children's Oncology Network of Bavaria (KIONET), pediatric patients have also been discussed on the board.
Contact
Dr. Lena Weiss, LMU Klinikum München
Organizational matters
The virtual ECTU board takes place once a month.
General questions about the process: BZKF_ECTU_Koordination@ukw.de
If you are interested in presenting a patient to the ECTU Tumor Board, please contact us at bzkf.ectu-Board@med.uni-muenchen.de
ECTU Tumor Board liaison physicians
Augsburg:
Liaison physician: Dr. med. David Graumann; David.Graumann@uk-augsburg.de
ECTU: Dr. med. Maximilian Schmutz; Maximilian.Schmutz@uk-augsburg.de
Erlangen:
Liasion doctor: Dr. Sophie Eckstein; sophie.eckstein@uk-erlangen.de
ECTU: Prof. Dr. Silvia Spörl; silvia.spoerl@uk-erlangen.de
Central email address: ectu.ccc@uk-erlangen.de
LMU:
Charlotte Schwicht; Charlotte.Schwicht@med.uni-muenchen.de
TUM:
Liasion doctor: Dr. med. Alisa Martina Lörsch; AlisaMartina.Loersch@mri.tum.de
Liasion doctor: Dr. med. David Schult-Hannemann; David.Schult-Hannemann@mri.tum.de
Liasion doctor/ECTU: Dr. med. Inga Hubrecht; Inga.Hubrecht@mri.tum.de
Central email address: ectu.board.cccm@mri.tum.de
Regensburg:
Liasion doctor: Dr. med. Christina Brummer; Christina.brummer@ukr.de
ECTU: Dr. med. Florian Lüke; florian.lueke@ukr.de
Central mail address: Christina.brummer@ukr.de
Würzburg:
Liasion doctor: Dr. med. Manik Chatterjee; Chatterjee_m@ukw.de
ECTU: Dr. med. Maria-Elisabeth Göbeler; Goebeler_M@ukw.de; Lena Schick; Schick_L@ukw.de


