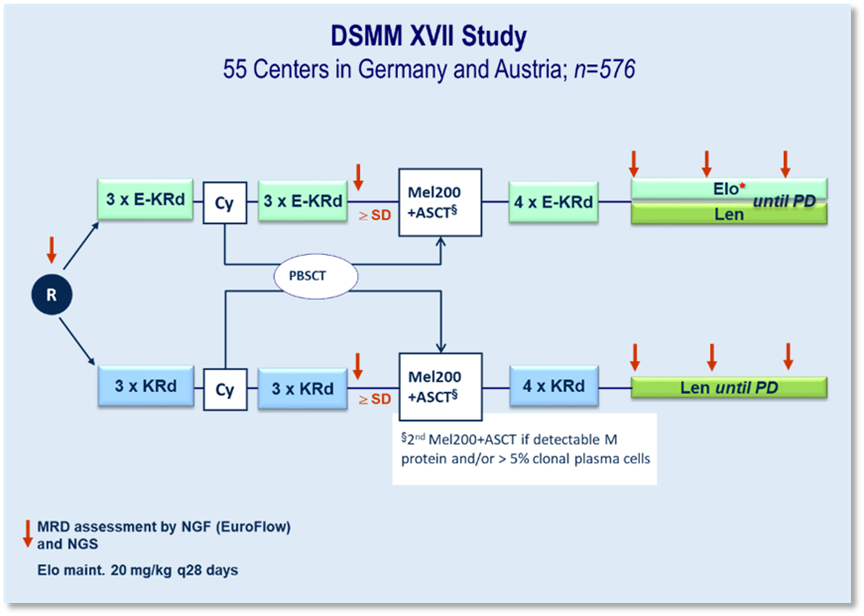
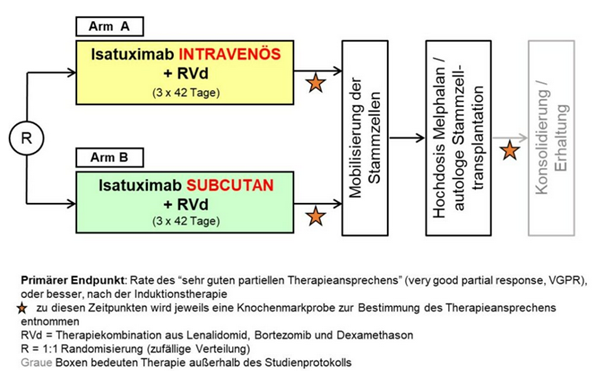
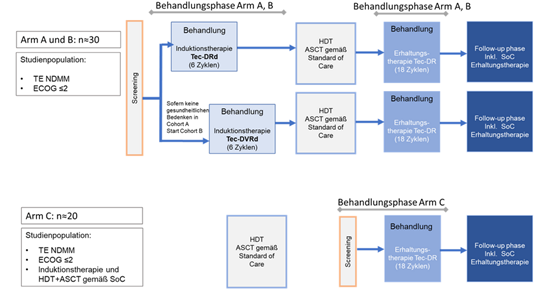
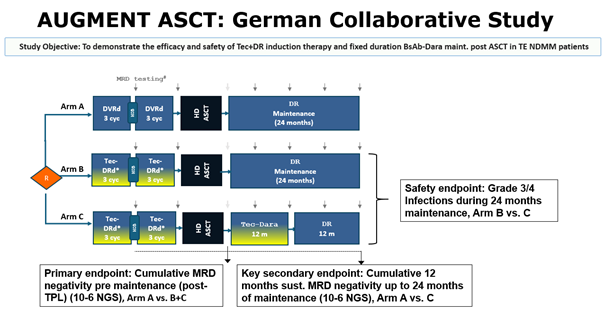
DSMM XVII
In the DSMM XVII study, a specific immunotherapy is being tested by our study group for the first time in phase 3. The humanized IgG4 antibody elotuzumab (Elo), which is directed against SLAMF7, is being used. This substance is being investigated in induction and consolidation together with carfilzomib (K), lenalidomide (R) and dexamethasone (d) against KRd. In maintenance, Elo-R will be tested against R.
Key characteristics:
" International phase 3 first-line study at 45 German and ten Austrian centers
" 576 patients (LPFV 10/2022)
The successful completion of a major cross-state inspection by the authorities in 2022/2023 confirms the integrity of the data and that the studies were conducted to high standards.
Initial results of the co-primary endpoint "rate of patients achieving VGPR or better and being MRD-negative (measured by next-generation flow with a sensitivity of 10-⁵) after six cycles of induction treatment" were published at ASCO 2003. This phase III study showed that the addition of elotuzumab to KRd (carfilzomib, lenalidomide and dexamethasone) improved the results of induction therapy in transplant-eligible patients with newly diagnosed multiple myeloma, particularly in terms of response rate and negative minimal residual disease (MRD).
At the beginning of the year, the next important co-primary endpoint "progression-free survival (PFS) after three years since randomization" was evaluated. This showed a statistically non-significant trend in favor of a better 36-month PFS for Elo-KRd.
DSMM XIX
Randomized phase 3 non-inferiority trial investigating induction therapy with lenalidomide, bortezomib and dexamethasone with intravenous or subcutaneous administration of isatuximab for patients with newly diagnosed multiple myeloma who are eligible for high-dose chemotherapy followed by autologous stem cell transplantation.
The GMMG-HD8/DSMM XIX study is a follow-up study to GMMG-HD7, which in turn aims to compare the success of treatment in newly diagnosed patients with and without the addition of the anti-CD38 monoclonal antibody isatuximab to the RVd combination (lenalidomide, bortezomib, dexamethasone).
In contrast, in the GMMG-HD8/DSMM XIX study, which started in March 2023, all patients (n=514) will receive the study drug isatuximab together with the RVd treatment regimen. The primary endpoint of the study is the non-inferiority of subcutaneous isatuximab administration compared to intravenous isatuximab, which is currently approved for the treatment of refractory myeloma, in terms of treatment response rates ("very good partial response or better"). Another endpoint of the study is the comparison of quality of life in the two study arms using PRO questionnaires.
Patients are randomized to the two arms and stratified according to R-ISS stage and body weight. A special feature of the study is the use of a medical device - a mechanical injector with which the patients are administered isatuximab subcutaneously, which is why GMMG-HD8/DSMM XIX is a combined AMG/MPDG study. In contrast to GMMG-HD7, GMMG-HD8/DSMM XIX ends after the first high-dose therapy/stem cell transplantation; maintenance therapy takes place outside the study.
GMMG-HD8/DSMM XIX is the first joint phase III trial with the German Multiple Myeloma Study Group (DSMM). In addition, the study will be conducted in two countries, Germany and Austria. Initially, the study was only launched in Germany, but trial sites in Austria have been added successively since the end of 2023. Recruitment of 514 study patients was successfully completed in March of this year.
Study design: Prospective, multicentre, randomized, unblinded phase III study for first-line therapy of transplantable MM patients up to 70 years of age
DSMM XVIII
Phase III study to investigate maintenance therapy with Iberdomid versus Iberdomid plus s.c. isatuximab after autologous hematopoietic stem cell transplantation for patients with newly diagnosed multiple myeloma.
This GMMG-HD9/DSMMXVIII study is designed as a follow-up treatment (maintenance therapy) to the GMMG-HD8/DSMM XIX study, which combines lenalidomide, bortezomib and dexamethasone with the monoclonal antibody isatuximab (Sarclisa®) in an induction therapy regimen. The GMMG-HD9/DSMM XVIII trial is aimed at patients who have successfully completed this induction therapy, followed by high-dose therapy and autologous stem cell transplantation, and have shown at least a partial response to treatment.
In this study, the drug Iberdomid is used in both arms, with arm B additionally receiving subcutaneous administration of isatuximab. Iberdomide has a similar effect to lenalidomide, but initial study results indicate that it is better tolerated and more effective, particularly in cancer cells that have become resistant to lenalidomide. The combination with isatuximab could lead to greater efficacy of the therapy and a longer time to relapse of the disease.
The aim of the study is to test whether the combination of iberdomide and isatuximab (arm B) is superior to iberdomide alone (arm A).
DSMMXX
Phase 2 study to evaluate the safety and efficacy of teclistamab in combination with daratumumab, lenalidomide and dexamethasone with and without bortezomib as induction therapy and teclistamab in combination with daratumumab and lenalidomide as maintenance therapy in transplant-eligible patients with newly diagnosed multiple myeloma.
The GMMG-HD10/DSMM XX trial, which started in November 2022, is a joint phase 2 trial of the GMMG and the German Multiple Myeloma Study Group (DSMM) in which a five-drug combination is being tested in induction therapy. An innovation of the study is the addition of a second antibody, teclistamab, to the combination of daratumumab (Dara), lenalidomide (R), bortezomib (V) and dexamethasone (d). Teclistamab (Tec) is a bispecific antibody that binds to the CD3 receptor complex on the surface of T cells and to the protein BCMA (B-cell maturation antigen) on the surface of myeloma cells. Binding to the CD3 complex leads to activation of the T cell, which then kills the myeloma cell bound to the other arm of the antibody. Because of its ability to "capture" T-cells, teclistamab is referred to as a T-cell engager.
The study design is divided into three study arms, with arm A and B receiving induction therapy with Tec-Dara-Rd (arm A) or Tec-Dara-VRd (arm B). After high-dose chemotherapy followed by autologous stem cell transplantation (HDT+ASCT) according to the standard of care, maintenance therapy with Tec-Dara-R will be carried out in arms A and B.
Arm C investigates maintenance therapy with Tec-Dara-R following induction, HDT+ASCT and, if necessary, consolidation therapy according to SOC
Approximately n=30 participants are planned for arms A and B and n=20 participants for arm C.
The primary study objective for arms A and B is to determine the safety and tolerability of teclistamab in combination with Dara-Rd or Dara-VRd as induction therapy, as well as teclistamab in combination with Dara-R as maintenance therapy after standard HDT+ASCT in newly diagnosed patients. The frequency and severity of adverse events will be determined. The secondary objectives include determining the efficacy of the respective combination therapy, rates of MRD negativity, treatment response and progression-free survival.
The treatment duration of the induction phase is 6 cycles (28 days each). For safety reasons, arm A (Tec-Dara-Rd) will be started first. If patient safety is ensured, patients are included in arm B (Tec-Dara-VRd), in which additional bortezomib treatment is planned.
After 6 cycles of induction therapy, high-dose chemotherapy + ASCT according to standard of care will follow. After completion of ASCT, the maintenance phase of the study begins under Tec-Dara-R with a duration of 18 cycles.
Study arm C begins with screening after completion of high-dose chemotherapy + ASCT according to the standard of care. Treatment is the same as in arms A and B with Tec-Dara-R as maintenance therapy for 18 cycles.
The follow-up phase is reached after "end of treatment" (EOT) or 18 cycles of maintenance therapy. During the follow-up, further therapies are allowed according to the investigator's decision and local standards.
AugMMent Study
AugMMenTE is a collaborative study between the "Deutsche Studiengruppe Multiples Myelom" (DSMM), the German-speaking Myeloma Multicenter Group (GMMG) (study sponsor University Hospital Heidelberg) and Johnson&Johnson Innovative Medicine (operational entity Medical Affairs Germany). AugMMenTE is an open-label, randomized interventional multicenter phase 3 clinical trial to assess the efficacy and safety of Tec+DR induction therapy and fixed duration Tec-D maintenance post autologous stem cell transplantation (ASCT) in transplant-eligible newly diagnosed Multiple Myeloma (TE NDMM) patients compared to the standard-of-care (SOC) PERSEUS regimen.
CMMC-Anima-2 Study
Circulating Multiple Myeloma Cells (CMMCs) Assay as a Non-Invasive tool for Minimal Residual Disease (MRD) Assessment in peripheral blood: A comparative study with bone marrow MRD
Acronym: CMMC-ANIMA - 02
Principal Investigator: Prof. Dr. Hermann Einsele (University Hospital Würzburg)
Document: Protocol
Version: #2.0
Draft date: 01-20-2021
Number of pages: 53
| Title | Circulating Multiple Myeloma Cells (CMMCs) Assay as a Non-Invasive tool for Minimal Residual Disease (MRD) Assessment in peripheral blood: a comparative study with bone marrow MRD |
| Acronym | CMMC-ANIMA-02 |
| PI | Prof. Dr. Hermann Einsele (Department of Medicine II, University Hospital Würzburg) |
| Sponsor | University Hospital Würzburg |
| Central Laboratory | Prof. Dr. Dr. Andreas Beilhack Laboratory, (Department of Medicine II, University Hospital Würzburg) |
| Collaborator | Menarini Silicon Biosystems SpA, BO Italy |
| Collaborator Clinical Project Leader | Dr. Delphine Chabut Global Medical Affairs Head Menarini Silicon Biosystems S.p.A. Via Giuseppe di Vittorio 21/B3, I-40013 Castel Maggiore (BO) Italia E-mail:dchabut@menarini.ch |
| Collaborator Medical Study Manager | Dr. Francesco Picardo Medical Affairs Manager Menarini Silicon Biosystems S.p.A. Via Giuseppe di Vittorio 21/B3, I-40013 Castel Maggiore (BO) Italia E-mail: fpicardo@siliconbiosystems.com |
| Background | The emergence of several new effective drugs over the recent past dramatically improved patient outcomes in Multiple Myeloma (MM), prolonging the median survival by 4 years and more [1, 2]. Complete response (CR) rates have increased in parallel, as well as the need to develop more sensitive methods to define deeper responses and monitor minimal residual disease (MRD). Minimal Residual Disease (MRD) refers to the small number of cancer cells that remain in the body after treatment. In multiple myeloma, MRD detection is crucial for assessing the depth of response to treatment and predicting patient outcomes. Furthermore, MRD assessment is of paramount importance for future prognosis, treatment monitoring, and MRD-driven treatment strategies. Measurement of MRD in bone marrow by both next-generation sequencing (NGS) of variable diversity joining (V(D)J) rearrangements or next-generation flow cytometry (NGF) is highly predictive of survival in MM [3,4] and may therefore represent a biomarker to adapt treatment strategies [5,6]. However, serial assessments of MRD involve repeated sampling, which implies the invasiveness and the risks of repeated BM aspirations. A different way of capturing tumor heterogeneity and potentially decide upon treatment over the course of time in a minimally invasive method in MM patients may be the use of liquid biopsies from peripheral blood. Menarini Silicon Biosystems developed an automated assay to enumerate and characterize Circulating Multiple Myeloma Cells (CMMCs) from peripheral blood of patients with plasma cell disorders. Briefly, a sample of 10 ml peripheral blood is collected in the CellRescue, a dedicated tube that preserves CMMC stabilizing CD138 expression at room temperature for 120 hours. A 4 ml aliquot is placed in the Celltracks Autoprep for automated sample preparation which includes the addition of anti-CD138 ferrofluid conjugated (capture antibody) and anti-CD38 (detection antibody), DAPI and anti-CD45/CD19 (exclusion antibody). Then, the sample is transferred to Celltracks Analyzer II where a proprietary software will provide to the operator a gallery of images of "events" to be reviewed. An event is then classified as CMMC if is CD38+, DAPI+, CD45/CD19-. The results will be provided as "CMMC/4ml". [7] So far, no peripheral blood assay to evaluate MRD has been validated. Both NGS and Euroflow methods in blood have shown low sensitivity with respectively, 44% and 40% of false negative in respect of bone marrow assessment. [8,9] CMMC assay potentially can detect down to 1 cell/4 ml, equivalent approximately to a sensitivity of 1 x 10-7 cell, higher than NGS (10-6) and NGF (10-6). Thus, our aim is to compare the CMMC assay performance in blood towards Euroflow NGF (mandatory) and Adaptive Clonoseq (optional) methods in bone marrow which are considered the reference standard for MRD assessment. |
| Objectives | Primary Objective: To contribute to set up a pooled dataset, with multiple sources, aimed to:
Secondary Objectives:
Exploratory Objectives:
|
| Collaboration design and needed data | The PB sample for CMMC enumeration is obtained within 3 days of BM collection for MRD assessment, if possible before BM aspiration to avoid the potential effects of the bone marrow procedure on the peripheral blood samples. EuroFlow data (mandatory), ClonoSEQ data (optional), mass spectrometry data (optional), ctDNA/cmmDNA data (optional) and CTCs (optional) will be collected at baseline and at first MRD assessment. Intermediate and final reports on data analyses and other specific analyses conducted at Fraunhofer ITEM and BZKF sites will be shared between parties. EuroFlow will be performed at Würzburg University Hospital when MRD and CTCs will be assessed. Mass spectrometry will be performed at Würzburg University Hospital when MRD will be assessed. ctDNA/cmmDNA analysis will be performed at Augsburg University Hospital when MRD will be assessed. CMMC enumeration using CellSearch system will be performed at Systemic Cancer Progression Laboratory (SCPL) of University of Regensburg at the specified points in time (see fig.1). CMMC isolation and single cell processing or archiving of the samples in glycerol will be performed at Fraunhofer ITEM Regensburg. |
| Inclusion Criteria |
|
| Methods | 200 patients including NDMM and RRMM patients will be enrolled into the CMMC-ANIMA-02 protocol. Patients assessed for MRD with BM aspirate analyzed with NGF EuroFlow (mandatory) and clonoSEQ (optional) assay will undergo additional PB sampling of 10 ml in CellRescue tubes and 27 ml in EDTA tubes within +3days from BM aspiration, preferably before BM aspiration and will be included in CMMC-ANIMA analysis as per primary objective of CMMC-ANIMA-02. PB samples for CMMCs analyses will be analyzed in the SCPL, University of Regensburg, with CMMCs assay in conjunction with CellSearch System. CellSearch image galleries will be provided to MSB for blinded central analysis, further research analysis and cut off definition. In case of discrepancy, an arbiter will decide the effective value of the CMMC enumeration. After completion of the CellSearch™ CMMC test, the enriched cells will be recovered and stored in glycerol at -20°C or isolated for single cell whole genome amplification. As detailed in the contract with Fraunhofer ITEM-Regensburg, the following selection criteria for subsequent cell isolation and single cell whole genome amplification (WGA) of CMMCs will be applied:
Samples with less than 5 cells will be considered unsuited for cell isolation and will not be processed further. Cells will be stored for no longer than 5 years from study completion. Detection of paraprotein by mass spectrometry in PB and NGF CTC assays, will be performed at Würzburg University Hospital according to standardized protocols when MRD will be assessed. Detection of ctDNA/cmmDNA in PB will be performed by CAPP-Seq at Augsburg University Hospital according to standardized methods when MRD will be assessed.
|
| Full data set Endpoints | Data collected from this CMMC-ANIMA-02 will be pooled with other sources to determine the following endpoints:
|
| Sample Size |
|
| Sites | Partner Sites Bavarian Center for Cancer Research (BZKF): University Hospital Würzburg University Hospital, TU Munich University Hospital Augsburg University Hospital Regensburg University Hospital Erlangen University Hospital, LMU Munich University of Regensburg Fraunhofer ITEM Regensburg |
| Study duration | up to the recruitment of 200 patients, maximum duration 2 years |
| Statistical Analysis | Data results of overall and BZKF/Fraunhofer specific analysis will be shared between parties. The comparison between CMMC assay and BM-MRD will be assessed as follows:
Furthermore, correlation among CMMC number with BM% plasma cell infiltration, M spike level and K/L ratio measured within 3 days before or after PB sampling will also be evaluated. Statistical analyses for exploratory endpoints including mass spectrometry, ctDNA/cmmDNA and NGF-CTCs will be performed based on individual requirements primarily interrogating
|
- Advanced diagnostic technologies for the highly sensitive detection of malignant plasma cells in bone marrow and peripheral blood in multiple myeloma.
- Predicting treatment response and disease progression by assessing the phenotype of normal and malignant plasma cells and their interaction with the tumor microenvironment in multiple myeloma.
- Prognostic significance of circulating tumor plasma cells in multiple myeloma.
- 3D patient in vitro models to test and validate the efficacy of therapies with the aim of improving personalized treatment strategies in multiple myeloma.
- The implementation of a strong network combining advanced 3D patient systems, innovative platforms for the investigation of immunotherapies and state-of-the-art analytical technologies, bringing together the unique expertise and resources of different BZKF centers, supported by the LT OMICS, LT Preclinical Models, LT Cellular Immunotherapies and the BZKF MM Study Group.
- Networking of the BZKF Center with industry partners for the validation and implementation of new diagnostic technologies for multiple myeloma. The BZKF currently has an active cooperation with the company Menarini Silicon Biosystems to compare various diagnostic technologies in bone marrow and peripheral blood in patients with multiple myeloma.
Establishment of a BZKF biobank-MM with objectives:
- Analysis of the significance of elotuzumab in the composition of the BM micromilieu
- Understanding the composition of B-cell populations in bone marrow with achieved MRD- ve CR
- Comparison of MRD techniques: NGF versus NGS
- Analysis of the expression duration of the target antigen SLAMF-7
- Use of a therapy directed against BCMA with CAR T cells in high-risk myeloma as a component of first-line therapy
- Understanding the persistence of resistance mechanisms against CARTs
Further study groups
- Acute myeloid leukemia (AML)
- Cancer of Unknown Primary (CUP)
- Endocrine and neuroendocrine tumors
- Head and neck tumors
- Liver carcinoma
- Lung tumors
- Lymphoma
- Malignant melanoma
- Breast carcinoma
- Ovarian carcinoma
- Pancreatic carcinoma
- Primary and secondary malignant brain tumors
- Prostate carcinoma
- R/R ALL
- Urothelial carcinoma
- Soft tissue sarcomas
- CNS tumors in children and adolescents