Standardized, harmonized diagnostics
Cancer of unknown primary (cancer of unknown origin) study group
Cancer of unknown origin is an entity with an internationally vague definition. In Germany, this entity is generally defined as a histologically confirmed malignancy with an unknown primary tumor after completion of standardized primary diagnostics. In practice, however, uniform standards regarding the extent of imaging and (molecular) pathological diagnostics are often not adhered to. The aim of the BZKF CUP Study Group (SG CUP) is therefore to improve the care of CUP patients on the basis of standardized, harmonized diagnostics and clear definitions. To ensure this, the study group has set up a CUP register to support these efforts.
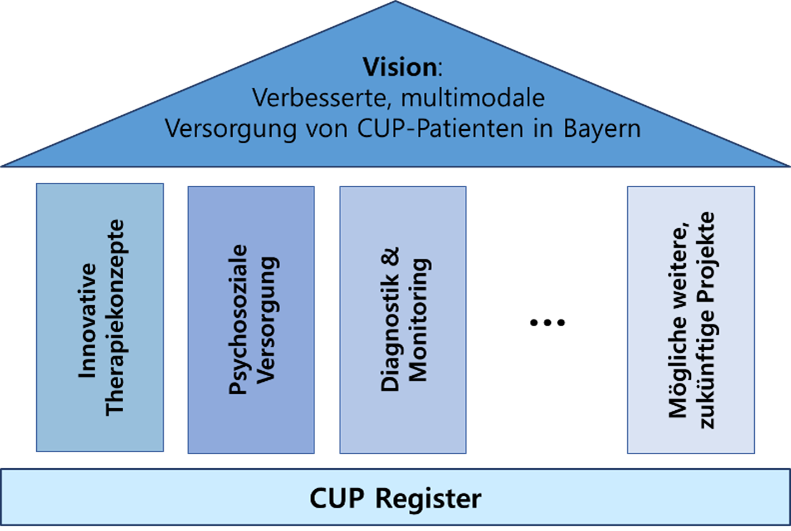
To this end, the CUP study group has developed a multimodal concept based on the CUP register (see Figure 1). Several pillars/fields of action are built on this basis, in which various approaches to improving the care of CUP patients are to be pursued. In addition to the development of innovative therapy concepts and novel diagnostics, the current focus will be on patient-oriented psychosocial care for CUP patients in Bavaria. Other pillars may be added to the concept at a later date.
To date, there is no comparable multimodal approach in Germany, nor are there any known research projects in Germany that shed light on the psychosocial care of CUP patients. The latter seems necessary, as CUP patients appear to have a particular psychological burden compared to other cancer patients due to their diagnosis, the lack of understanding of the cancer and the associated uncertainty of the disease. Accordingly, there may be increased needs such as a greater need for information and additional support.
Over the next few years, the SG CUP will therefore focus on a research project in the area of patient-oriented psychosocial care. A scientifically based survey will be carried out to determine and analyze the needs and psychosocial burdens of CUP patients and their relatives in Bavaria and, based on this, develop strategies to improve care for this patient group.
Another funded project is the development of an innovative therapy concept that investigates the use of ex vivo drug screening in CUP. There is close cooperation here with the Omics, Genomics and Liquid Biopsy lighthouse as well as the Institute for Experimental Medicine and the Fraunhofer Institute in Regensburg.
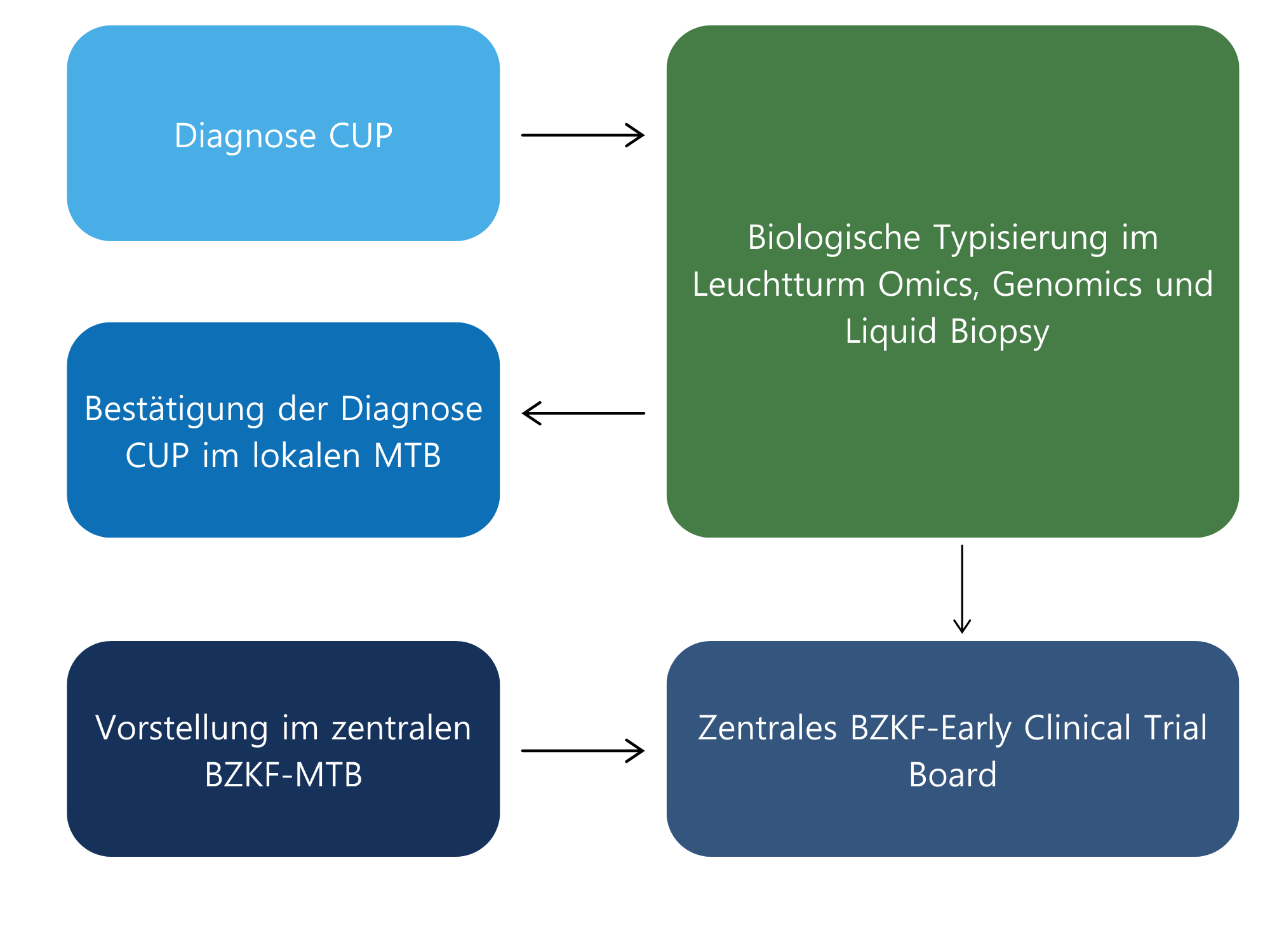
The BZKF network is used for the clinical testing of other innovative therapies (including those developed outside the BZKF) in early clinical trials. A mandatory prerequisite is confirmation of the diagnosis of CUP in the local molecular tumor board (MTB, see Figure 2):
Psychosocial needs analysis, including the identification and evaluation of psychological stress in CUP patients
Creation of input masks and a register database with the help of OnkoStar; collection, evaluation and presentation of retrospective data
Agreement on a clear definition of CUP, determination of the associated standard diagnostics and harmonization
Prospective data collection in the registry
Collection of tissue and blood samples, development of the biomaterial collection
Comprehensive Bavaria-wide CUP register as a basis for scientific projects
Development of patient-oriented care for CUP patients as an example of best practice within Germany based on the psychosocial needs analysis
Multimodal and thus improved care for CUP patients in Bavaria
Establishment of evidence-based, suitable measures (therapy concepts, psychosocial care, etc.)
Continuation of networking with other BZKF working groups and lighthouses with the aim of improving diagnostic and therapeutic options
Further study groups
- Acute myeloid leukemia (AML)
- Endocrine and neuroendocrine tumors
- Head and neck tumors
- Liver carcinoma
- Lung tumors
- Lymphoma
- Malignant melanoma
- Multiple myeloma
- Breast cancer
- Ovarian carcinoma
- Pancreatic carcinoma
- Primary and secondary malignant brain tumors
- Prostate carcinoma
- R/R ALL
- Urothelial carcinoma
- Soft tissue sarcomas