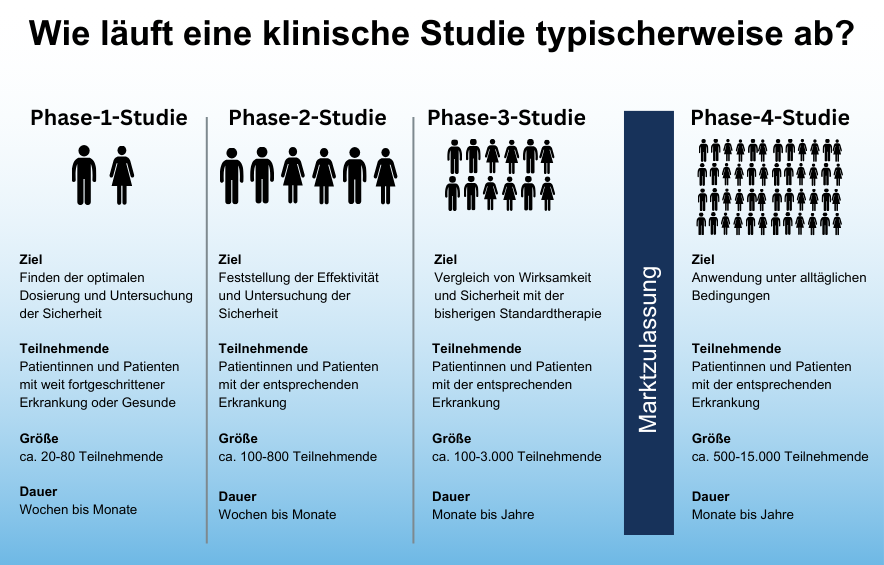
How safe is participation?
Patient safety is always our top priority! Every clinical trial has already gone through a long process with many interim controls before it starts. Only when the benefits and risks of a new treatment procedure are in a presumably favorable relationship to each other is its use in humans even considered. In addition, the safety of a clinical trial is guaranteed by strict legal regulations.
Participation can only take place after careful examination and approval by the attending physician. Participation in a study is always voluntary and can be withdrawn at any time without giving reasons. All information collected is subject to data protection.
Clinical trials offer ...
- Access to the latest targeted therapy options, which are expected to be more effective/tolerable
- Comprehensive information, individual support and particularly intensive monitoring
- The opportunity to make an active personal contribution to medical progress
What could be the disadvantages of participating in a trial?
- Less favorable efficacy and side effect profile than expected
- More time and additional examinations
About the early study decision aid
The decision aid is aimed at people who have cancer or a blood disorder and are faced with the decision to take part in an early clinical trial.
How are clinical trials organized at the BZKF sites?
Clinical trials on patients with certain characteristics of cancer are carried out by so-called clinical study groups. They can be grouped according to a specific methodology or focus on the treatment of a disease at a specific stage. However, they are often a mixture of both. The promotion of clinical study groups with the aim of increasing the quality of care for patients in Bavaria is one of the most important concerns of the Bavarian Center for Cancer Research. The focus here is on the rapid integration of new knowledge into the everyday care of patients.
Everything about early clinical trials (Early Clinical Trial Units, ECTUs)
You can find an overview of all ongoing studies in the
Podcast: Clinical trials - What do I need to know about them as a patient?
Clinical trials are a central component of modern cancer research and offer patients access to innovative therapeutic approaches.
In this podcast episode, study coordinator Silvia Müller and Dr. Carlo Maurer from the CCC Munich talk about this important topic. They describe the structure of clinical trials, how patients can take part in a clinical trial and the benefits of participating in a trial.
Find out more about the exciting world of clinical trials and how they make a valuable contribution to medicine.

What do I have to do if I want to take part in a study?
Interested patients should first talk to their treating doctor. There are certain criteria that must be taken into account for every clinical trial and which determine whether participation is possible. Before making a decision, patients should carefully weigh up the opportunities and risks.
Telephone cancer counseling
You can also obtain further general information on the subject of cancer therapy via the free BürgerTelefonKrebs: 0800 85 100 80.
Our staff will be happy to answer your questions and help you!